Adverse Events Data Model
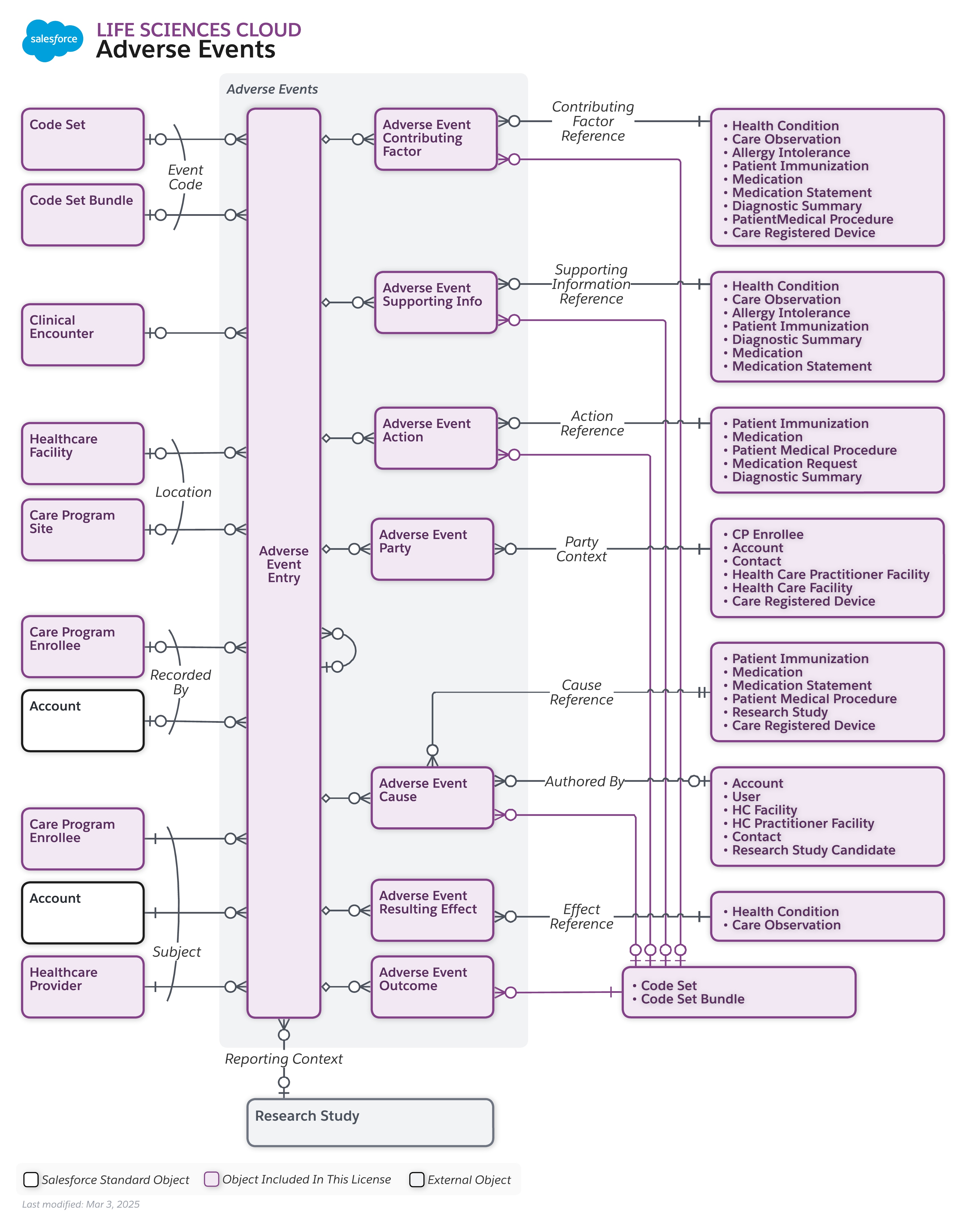
To view a higher-resolution version of this diagram, right-click (or Control-click on Mac) and select "Open Image in New Tab."
Entities and relationships used to manage and document any unfavorable signs, symptoms, or diseases that occur in participants during a research study.
Account, Adverse Event Action, Adverse Event Cause, Adverse Event Contributing Factor, Adverse Event Entry, Adverse Event Outcome, Adverse Event Party, Adverse Event Resulting Effect, Adverse Event Supporting Info, Care Program Enrollee, Care Program Site, Clinical Encounter, Code Set, Code Set Bundle, Healthcare Facility, Healthcare Provider, Research Study