Participant Management Data Model
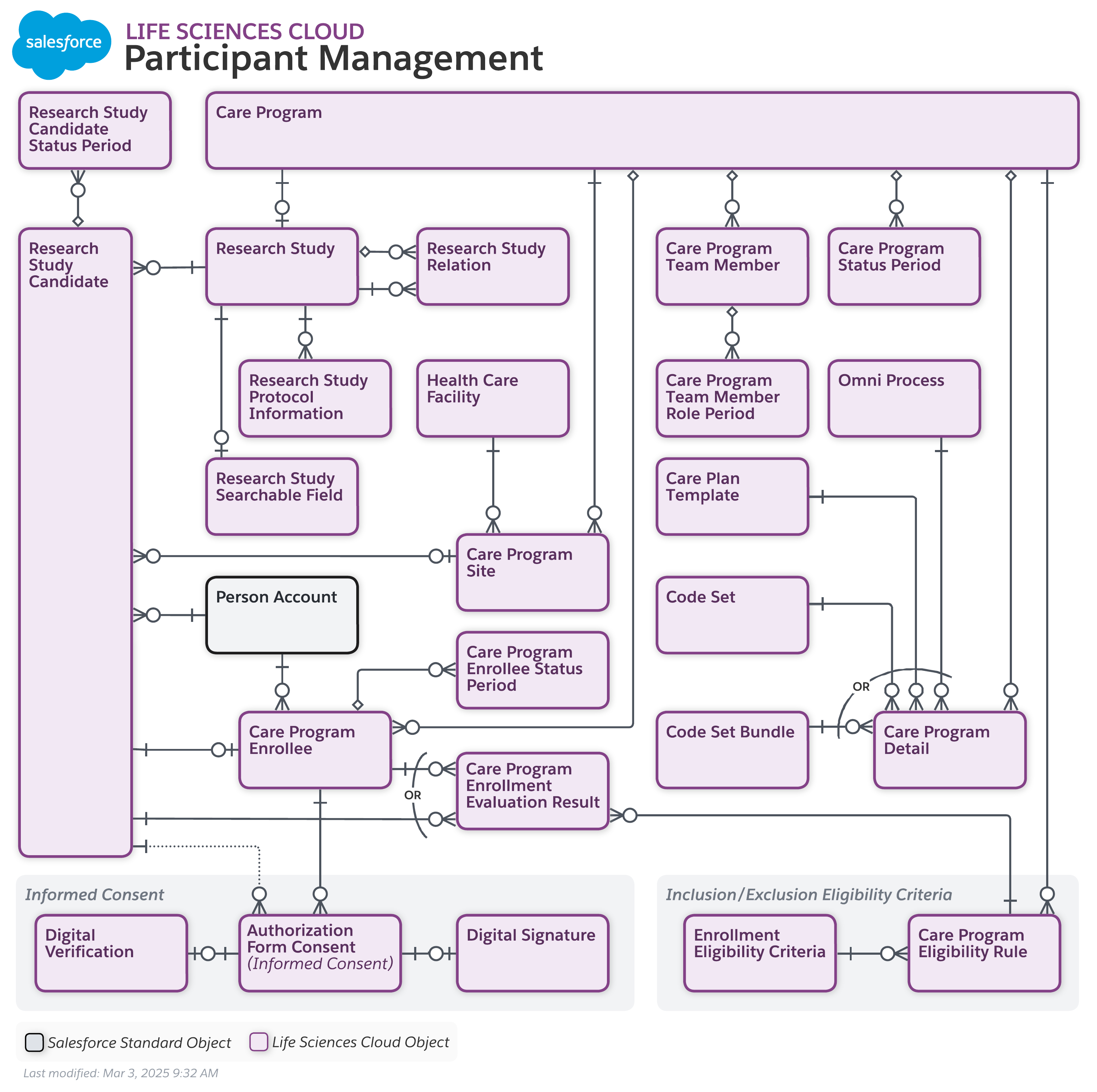
To view a higher-resolution version of this diagram, right-click (or Control-click on Mac) and select "Open Image in New Tab."
Entities and relationships allowing streamlined recruitment and enrollment processes in clinical trials with advanced digital solutions – including storing clinical trial data representing care programs and research studies
Authorization Form Consent, Care Plan Template, Care Program, Care Program Detail, Care Program Eligibility Rule, Care Program Enrollee, Care Program Enrollee Status Period, Care Program Enrollment Evaluation Result, Care Program Site, Care Program Status Period, Care Program Team Member, Care Program Team Member Role Period, Code Set, Code Set Bundle, Digital Signature, Digital Verification, Enrollment Eligibility Criteria, Health Care Facility, Informed Consent, Omni Process, Person Account, Research Study, Research Study Candidate, Research Study Candidate Status Period, Research Study Protocol Information, Research Study Relation, Research Study Searchable Field